Resumo
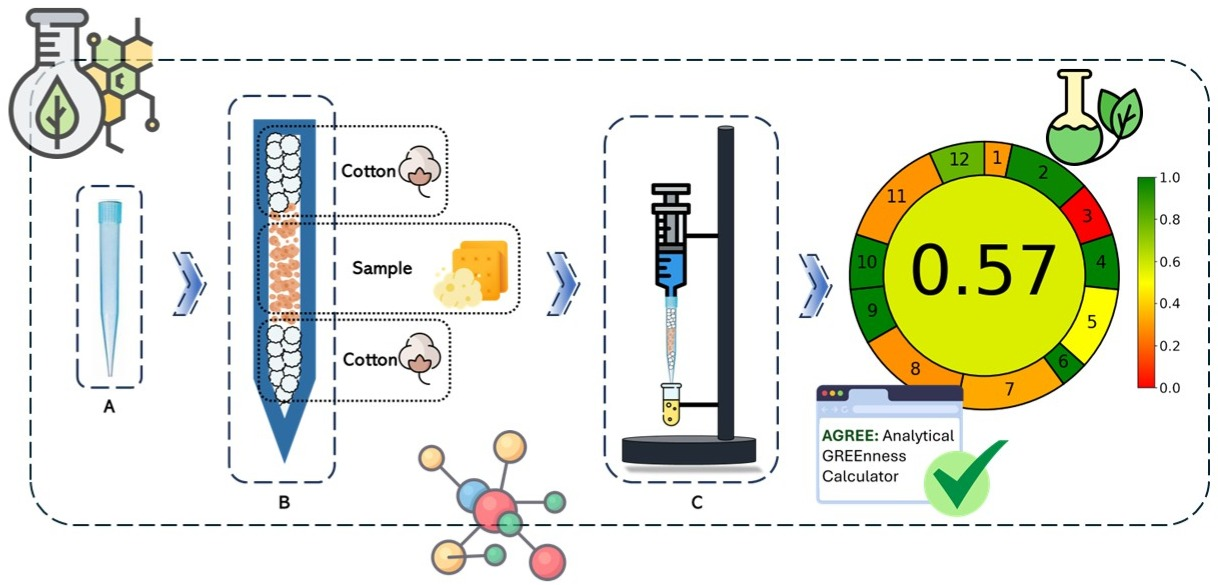
Lipids are essential biological molecules that influence aroma, flavor, and human health; however, excessive consumption can lead to diseases such as cardiovascular problems. Monitoring lipid content in food is crucial, and the traditional Soxhlet method is commonly used for lipid extraction. However, this method requires large amounts of petroleumbased solvents, energy, and time, making it incompatible with principles of Green Chemistry. To address this issue, a novel, environmentally friendly solid-liquid extraction method using disposable pipette tips (SLE-DT) was developed for lipid microextraction from food samples. The method was developed using a 22 full factorial design with salty crackers, identifying a 100 mg sample and 10 extraction cycles as the enhanced experimental condition. The SLE-DT method was tested on ten biscuit samples, showing lipid content consistent with product labels. Compared to the traditional Goldfish method, SLE-DT was significantly greener, scoring 0.56 versus 0.29, aligning better with Green Chemistry principles.
Referências
1. Caetano GAO, Fonseca AA, Figueiredo CB. Teor e composição de lipídeos como ferramenta de gestão na nutrição de bovinos. Res Soc Dev. 2020;9(7):e334974037. https://doi.org/10.33448/rsd-v9i7.4037
2. Wang D, Xiao H, Lyu X, Chen H, Wei F. Lipid oxidation in food science and nutritional health: a comprehensive review. Oil Crop Sci. 2023;8(1):35-44. https://doi.org/10.1016/j.ocsci.2023.02.002
3. Tan K, Lim L, Peng Y, Cheong KL. Effects of food processing on the lipid nutritional quality of commercially important fish and shellfish. Food Chem X. 2023;20:101034. https://doi.org/10.1016/j.fochx.2023.101034
4. AlMohamadi H, Alamoudi M, Yameen MZ, Naqvi SR. An integrated approach for the extraction of lipids from marine macroalgae consortium using RSM optimization and thermo-kinetic analysis. Chemosphere. 2023;338:139623. https://doi.org/10.1016/j.chemosphere.2023.139623
5. Thilakarathna RCN, Siow LF, Tang TK, Chan ES, Lee YY. Physicochemical and antioxidative properties of ultrasound-assisted extraction of mahua (Madhuca longifolia) seed oil in comparison with conventional Soxhlet and mechanical extractions. Ultrason Sonochem. 2023;92:106280. https://doi.org/10.1016/j.ultsonch.2022.106280
6. Fuente B, Pinela J, Mandim F, Heleno SA, Ferreira ICFR, Barba FJ et al. Nutritional and bioactive oils from salmon (Salmo salar) side streams obtained by Soxhlet and optimized microwave-assisted extraction. Food Chem. 2022;386:132778. https://doi.org/10.1016/j.foodchem.2022.132778
7. International Union of Pure and Applied Chemistry. IUPAC. Standard methods for the analysis of oils, fats and derivatives. 1987. 7th Edition, 1st Supplement. Available from: https://old.iupac.org/publications/books/ISBN0632033371_compress.pdf
8. Brum AAS, Arruda LF, Regitano-d’Arce MAB. Métodos de extração e qualidade da fração lipídica de matérias-primas de origem vegetal e animal. Quim Nova. 2009;32(4):849-54. https://doi.org/10.1590/S0100-40422009000400005
9. Castro MDL, García-Ayuso L. Soxhlet extraction of solid materials: an outdated technique with a promising innovative future. Anal Chim Acta. 1998;369(1-2):1-10. https://doi.org/10.1016/S0003-2670(98)00233-5
10. Forfang K, Zimmermann B, Kosa G, Kohler A, Shapaval V. FTIR spectroscopy for evaluation and monitoring of lipid extraction efficiency for oleaginous fungi. PLoSONE. 2017;12(1):e0170611. https://doi.org/10.1371/journal.pone.0170611
11. Anastas PT, Warner JC. Green chemistry: theory and practice. Oxford University PressOxford; 2000, online edn. https://doi.org/10.1093/oso/9780198506980.001.0001
12. Anastas P, Eghbali N. Green chemistry: principles and practice. Chem Soc Rev. 2010;39(1):301-12. https://doi.org/10.1039/B918763B
13. Nebolisa NM, Umeyor CE, Ekpunobi UE, Umeyor IC, Okoye FB. Profiling the effects of microwaveassisted and soxhlet extraction techniques on the physicochemical attributes of Moringa oleifera seed oil and proteins. Oil Crop Sci. 2023;8(1):16-26. https://doi.org/10.1016/j.ocsci.2023.02.003
14. Ferreira BL, Beik JV, Alves SJZ, Henrique FA, Sauer E, Chornobai CA et al. Extração assistida por ultrassom para determinação de lipídeos em alimentos: um experimento de laboratório. Quim Nova. 2020;43(9):1320-5. https://doi.org/10.21577/0100-4042.20170592
15. Devineni N, Mallikarjunan P, Chinnan MS, Phillips RD. Supercritical fluid extraction of lipids from deep-fried food products. J Am Oil Chem Soc. 1997;74(12):1517-23. https://doi.org/10.1007/s11746-997-0070-8
16. Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE-Analytical GREEnness metric approach and software. Anal Chem. 2020;92(14):10076-82. https://doi.org/10.1021/acs.analchem.0c01887
17. Kozłowska M, Gruczyńska E, Ścibisz I, Rudzińska M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem. 2016;213(15):450-6. https://doi.org/10.1016/j.foodchem.2016.06.102
18. Ribeiro PPC, Silva DM, Dantas MM, Ribeiro KD, Dimenstein R, Damasceno KSFSC. Determination of tocopherols and physicochemical properties of faveleira (Cnidoscolus quercifolius) seed oil extracted using different methods. Food Sci Technol. 2019;39(2):280-5. https://doi.org/10.1590/fst.24017
19. Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. 1978;90(1):420-6. https://doi.org/10.1016/0003-2697(78)90046-5
20. Kumar RR, Rao PH, Arumugam M. Lipid extraction methods from microalgae: a comprehensive review. Front Energy Res. 2015;2:article61. https://doi.org/10.3389/fenrg.2014.00061
21. Barros CB. Validação de métodos analíticos. Biológico. 2002;64(2):175-7. Available from: http://www.biologico.agricultura.sp.gov.br/uploads/docs/bio/v64_2/barros.pdf

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Copyright (c) 2025 Bernardo Moura Zapelini , Caroline Gonçalves , Eduardo Sidinei Chaves, Bruno Luís Ferreira