Resumen
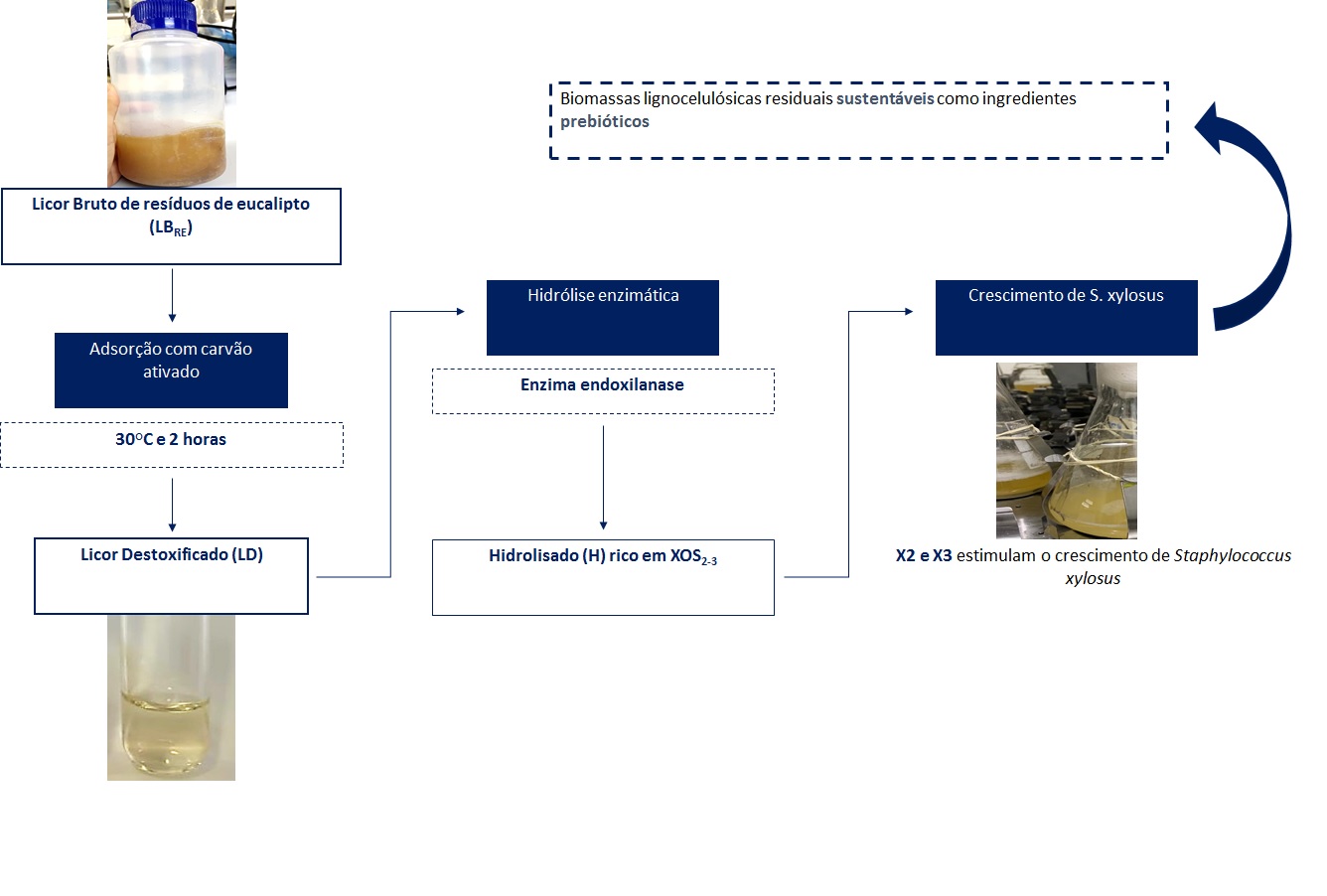
Xilooligossacarídeos (XOS) são reconhecidos pelo seu potencial prebiótico relevante para diversos setores industriais e foram obtidos após o pré-tratamento hidrotérmico da biomassa lignocelulósica residual de galhos de eucalipto. Subprodutos inibitórios são gerados durante o processo de solubilização dos oligossacarídeos e acabam comprometendo a utilização do licor em microrganismos. Neste trabalho, o processo de destoxificação, hidrólise enzimática e atividade estimulantes de crescimento da bactéria Staphylococcus xylosus foram estabelecidos. Os resultados mostraram que a adsorção com carvão ativado em pó removeu cerca de 55% do ácido acético e mais de 90% do ácido fórmico, compostos fenólicos, lignina solúvel, furfural e 5 hidroximetilfurfural, e que a soma dos oligossacarídeos xilobiose (X2) e xilotriose (X3) foram maximizadas de 0,57 g/L para 1,21 g/L com 110 U/gXOS da enzima endoxilanase e 6,3% do licor destoxificado na hidrólise enzimática. O consumo de cerca de 63% de X2 e de 46% de X3 pela bactéria em meio basal deficiente em fontes de carbono, mas acrescido com os oligômeros, proporcionou maior crescimento celular em relação aos meios basais com alta composição de carbono, com e sem XOS, revelando seu potencial prebiótico pelo efeito estimulante de crescimento.
Citas
1. Indústria Brasileira de Árvores – IBÁ. Relatório anual IBÁ 2021. [acesso 2022 Jul 18]. Disponível em: https://iba.org/datafiles/publicacoes/relatorios/relatorioiba2021-compactado.pdf utm_source=akna&utm_medium=email&utm_campaign=iba lanca-relatorio-anual-2021
2. Ávila PF, Martins M, Costa FAA, Goldbeck R. Xylooligosaccharides production by commercial enzyme mixture from agricultural wastes and their prebiotic and antioxidant potential. Bioact Carbohydr Diet Fibre. 2020;24:100234. https://doi.org/10.1016/j.bcdf.2020.100234
3. Ahmad N, Zakaria MR. Oligosaccharide from hemicellulose. Chapter 8. In: Ariffin H, Sapuan SM, Hassan MA, Editors. Lignocellulose for Future Bioeconomy, Elsevier; 2019.p.135-52. https://doi.org/10.1016/B978-0-12-816354-2.00008-61.1. Indústria Brasileira de Árvores – IBÁ. Relatório anual IBÁ 2021. [acesso 2022 Jul 18]. Disponível em: https://iba.org/datafiles/publicacoes/relatorios/relatorioiba2021-compactado.pdf utm_source=akna&utm_medium=email&utm_campaign=iba lanca-relatorio-anual-2021
2. Ávila PF, Martins M, Costa FAA, Goldbeck R. Xylooligosaccharides production by commercial enzyme mixture from agricultural wastes and their prebiotic and antioxidant potential. Bioact Carbohydr Diet Fibre. 2020;24:100234. https://doi.org/10.1016/j.bcdf.2020.100234
3. Ahmad N, Zakaria MR. Oligosaccharide from hemicellulose. Chapter 8. In: Ariffin H, Sapuan SM, Hassan MA, Editors. Lignocellulose for Future Bioeconomy, Elsevier; 2019.p.135-52. https://doi.org/10.1016/B978-0-12-816354-2.00008-6
4. Otieno DO, Ahring BK. A thermochemical pretreatment process to produce xylooligosaccharides (XOS), arabinooligosaccharides (AOS) and mannooligosaccharides (MOS) from lignocellulosic biomasses. Bioresour Technol. 2012;112:285-92 .https://doi.org/10.1016/j.biortech.2012.01.162
5. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ et al. The International scientific association for probiotics and prebioticos (ISAPP) consensus statement on the definition and of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491-502. https://doi.org/10.1038/nrgastro.2017.75
6. Jia H, Shao T, Zhong C, Li H, Jiang M, Zhou H et al. Evaluation of xylitol production using corncob hemicellulosic hydrolysate by combining tetrabutylammonium hydroxide extraction with dilute acid hydrolysis. Carbohydr Polym. 2016;151:676-83. https://doi.org/10.1016/j.carbpol.2016.06.013
7. Misra S, Raghuwanshi S, Saxena RK. Evaluation of corncob hemicellulosic hydrolysate for xylitol production by adapted strain of Candida tropicalis. Carbohydr Polym. 2013;92(2):1596-601. https://doi.org/10.1016/j.carbpol.2012.11.033
8. Cao R, Xu Y. Efficient preparation of xylonic acid from xylonate fermentation broth by bipolar membrane electrodialysis. Appl Biochem Biotechnol. 2019;187(1):396-406. https://doi.org/10.1007/s12010-018-2827-y
9. Antunes FAF, Santos JC, Chandel AK, Carrier DJ, Peres GFD, Milessi TSS et al. Repeated batches as a feasible industrial process for hemicellulosic ethanol production from sugarcane bagasse by using immobilized yeast cells. Cellulose. 2019;26(6):3787-800. https://doi.org/10.1007/s10570-019-02341-z
10. Nascimento BF, Araujo CMB, Nascimento AC, Silva FLH, Melo DJN, Jaguaribe EF et al. Detoxification of sisal bagasse hydrolysate using activated carbon produced from the gasification of açaí waste. J Hazard Mater. 2021;409:124494. https://doi.org/10.1016/j.jhazmat.2020.124494
11. Mussatto SI, Roberto IC. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour Technol. 2004;93(1):1-10. https://doi.org/10.1016/j.biortech.2003.10.005
12. van Zyl C, Prior BA, du Preez JC. Acetic acid inhibition of d-xylose fermentation by Pichia stipitis. Enzyme Microb Technol. 1991;13(1):82-6. https://doi.org/10.1016/0141-0229(91)90193-E
13. Arruda PV, Chaud LCS, Felipe MGA, Pivetta LR. Efeito da destoxificação do hidrolisado de bagaço de cana sobre a remoção de fenóis, a perda de açúcares e a bioconversão de xilose em xilitol. Nucleus. 2008;5(1):166-182. https://doi.org/10.3738/192-227869
14. Nascimento RF, Lima ACA, Vidal CB, Melo DQ, Raulino GSC. Adsorção: aspectos teóricos e aplicações ambientais. E-book. 2. ed. Fortaleza (CE): Impressa Universitária; 2020. [acesso em 2022 Jul 28]. Disponível em: http://www.repositorio.ufc.br/handle/riufc/53271
15. Bansal RC, Goyal M. Activated carbon adsorption. Roca Raton (NY): CRC Press; 2005. https://doi.org/10.1201/9781420028812
16. Ramos PH, Guerreiro MC, Resende EC, Gonçalves M. Production and characterization of activated carbon prepared from PVA defect coffee. Quím Nova. 2009;32(5):1139-43. https://doi.org/10.1590/s0100-40422009000500011
17. Yagmur E, Ozmak M, Aktas Z. A novel method for production of activated carbon from waste tea by chemical activation with microwave energy. Fuel. 2008;87(15-16):3278-85. https://doi.org/10.1016/j.fuel.2008.05.005
18. Costa-Trigo I, Paz A, Otero-Penedo P, Outeirino D, Oliveira RPS, Domínguez JM. Detoxification of chestnut burrs hydrolyzates to produce biomolecules. Biochem Eng J. 2020;159:107599. https://doi.org/10.1016/j.bej.2020.107599
19. Alves LA, Felipe MGA, Silva JBAE, Silva SS, Prata AMR. Pretreatment of sugarcane bagasse hemicellulose hydrolysate for xylitol production by Candida guilliermondii. Appl Biochem Biotechnol. 1998;70:89-98. https://doi.org/10.1007/BF02920126
20. Polizeli MLTM, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorin DS. Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol. 2005;67(5):577-91. https://doi.org/10.1007/s00253-005-1904-7
21. Zhang H, Han L, Dong H. An insight to pretreatment, enzyme adsorption and enzymatic hydrolysis of lignocellulosic biomass: experimental and modeling studies. Renew Sustain Energy Rev. 2021;140:110758. https://doi.org/10.1016/j.rser.2021.110758
22. Pesquisa Brasileira de Pesquisa Agropecuária – EMBRAPA. Xilanases microbianas e suas aplicações industriais. [acesso em 2022 Jul 28]. Disponível em: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1134008/1/Xilanases-microbianas-esuas-aplicac807o771es-industriais-2021.pdf
23. Brenelli LB, Figueiredo FL, Damasio A, Franco TT, Rabelo SC. An integrated approach to obtain xylooligosaccharides from sugarcane straw: from lab to pilot scale. Bioresour Technol. 2020;313:123637. https://doi.org/10.1016/j.biortech.2020.123637
24. Krutmann J. Pre- and probiotics for human skin. J Dermatol Sci. 2009;54(1):1-5. https://doi.org/10.1016/j.jdermsci.2009.01.002
25. Stavropoulou DA, Maere H, Berardo A, Janssens B, Filippou P, Vuyst L et al. Pervasiveness of Staphylococcus carnosus over Staphylococcus xylosus is affected by the level of acidification within a conventional meat starter culture set-up. Int J Food Microbiol. 2018;274:60-6. https://doi.org/10.1016/j.ijfoodmicro.2018.03.006
26. Egert M, Simmering R, Riedel CU. The association of the skin microbiota with health, immunity, and disease. Clin Pharmacol Ther. 2017;102(1):62-9. https://doi.org/10.1002/cpt.698
27. Leroy S, Lebert I, Andant C, Talon R. Interaction in dual species biofilms between Staphylococcus xylosus and Staphylococcus aureus. Int J Food Microbiol. 2020;326:108653. https://doi.org/10.1016/j.ijfoodmicro.2020.108653
28. Tamayo-Pena JA, Felix TC, Pacheco LCA, Brenelli LB, Gonçalves AR, Franco TT. BBEST 2020-21/ Biofuture Summit II. Dezembro de 2020; São Paulo.
29. Heylmann KKA, Lopes BV, Afonso TF, Demarco CF, Cadaval Junior TR, Quadro MS et al. Produção, caracterização e aplicação de carvão ativado de caroço de pêssego no tratamento de efluente têxtil. Eng Sanit Ambient. 2021;26(3):485-94. https://doi.org/10.1590/S1413-415220190226
30. Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426-8. https://doi.org/10.1021/ac60147a030
31. Rocha GJM, Gonçalves AR, Oliveira BR, Olivares EG, Rossell CEV. Steam explosion pretreatment reproduction and alkaline delignification reactions performed on a pilot scale with sugarcane bagasse for bioethanol production. Ind Crop Prod. 2012;35(1):274-9. https://doi.org/10.1016/j.indcrop.2011.07.010
32. Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D. Determination of sugars, byproducts, and degradation products in liquid fraction process samples: Laboratory Analytical Procedure (lLAP). Thecnical Report NREL/TP-510-42623; 2006. Disponível em: https://www.nrel.gov/docs/gen/fy08/42623.pdf
33. Lian Z, Wang Y, Luo J, Lai C, Yong Q, Yu S. An integrated process to produce prebiotic xylooligosaccharides by autohydrolysis, nanofiltration and endo-xylanase from alkali-extracted xylan. Bioresour Technol. 2020;314:123685. https://doi.org/10.1016/j.biortech.2020.123685
34. Antunes FAF, Thomé LC, Santos JC, Ingle AP, Costa CB, Anjos V et al. Multi-scale study of the integrated use of the carbohydrate fractions of sugarcane bagasse for ethanol and xylitol production. Renew Energy. 2021;163:1343-55. https://doi.org/10.1016/j.renene.2020.08.020
35. Wikandari R, Millati R, Syamsiyah S, Muriana R, Ayuningsih Y. Effect of furfural, hydroxymethylfurfural and acetic acid on indigeneous microbial isolate for bioethanol production. Agric J. 2010;5(2):105-9. https://doi.org/10.3923/aj.2010.105.109
36. Ge L, Zhao C, Chen S, Li Q, Zhou T, Jiang H et al. An analysis of the carbonization process and volatilerelease characteristics of coal-based activated carbon. Energy. 2022;257:124779. https://doi.org/10.1016/j.energy.2022.124779
37. Sujiono EH, Zabrian D, Zurnansyah, Mulyati, Zharvan V, Samnur et al. Fabrication and characterization of coconut shell activated carbon using variation chemical activation for wastewater treatment application. Results Chem. 2022;4:100291. https://doi.org/10.1016/j.rechem.2022.100291
38. Vargas DP, Giraldo L, Moreno-Piraján JC. Co2 adsorption on granular and monolith carbonaceous materials. J Anal App Pyrolysis. 2012;96:146-52. https://doi.org/10.1016/j.jaap.2012.03.016
39. Paniagua-García AI, Hijosa-Valsero M, Díez-Antolínez R, Sánchez ME, Coca M. Enzymatic hydrolysis and detoxification of lignocellulosic biomass are not always necessary for ABE fermentation : The case of Panicum virgatum. Biomass Bioenergy. 2018;116:131-9. https://doi.org/10.1016/j.biombioe.2018.06.006
40. Evstigneyev EI, Shevchenko SM. Structure, chemical reactivity and solubility of lignin: a fresh look. Wood SciTechnol. 2019;53:7-47. https://doi.org/10.1007/s00226-018-1059-1
41. Horvath AL. Solubility of structurally complicated materials: I. Wood. J Phys Chem Ref Data. 2006;35:77-92. https://doi.org/10.1063/1.2035708
42. Ahmedna M, Marshall WE, Rao RM. Granular activated carbons from agricultural by-products: preparation, properties, and application in cane sugar refining. Louisiana State University, LSU Digital Commons. Bulletin 869; 2000. p. 1-56. Disponível em: https://digitalcommons.lsu.edu/cgi/viewcontent.cgi?article=1038&context=agcenter_bulletins
43. Adekola FA, Oba IA. Biosorption of formic and acetic acids from aqueous solution using activated carbon from shea butter seed shells. Appl Water Sci. 2016;7(6):2727-36. https://doi.org/10.1007/s13201-016-0491-3
44. Pino MS, Rodríguez-Jasso RM, Michelin M, Flores-Gallegos A, Morales-Rodriguez R, Teixeira JA et al. Bioreactor design for enzymatic hydrolysis of biomass under the biorefinery concept. Chem Eng J.2018;347:119-36. https://doi.org/10.1016/j.cej.2018.04.057
45. Santibáñez L, Henr íquez C, Corro-Tejeda R, Bernal S, Armijo B, Salazar O. Xylooligosaccharides from lignocellulosic biomass: a comprehensive review. Carbohydr Polym. 2021;251:117118. https://doi.org/10.1016/j.carbpol.2020.117118
46. García-Ruiz A, Bartolomé B, Martínez-Rodríguez AJ, Pueyo E, Martín-Álvarez PJ, Moreno-Arribas MV. Potential of phenolic compounds for controlling lactic acid bacteria growth in wine. Food Control. 2008;19(9):835-41. https://doi.org/10.1016/j.foodcont.2007.08.018
47. Basak B, Jeon BH, Kim TH, Lee JC, Chatterjee PK, Lim H. Dark fermentative hydrogen production from pretreated lignocellulosic biomass: effects of inhibitory byproducts and recent trends in mitigation strategies. Renew Sustain Energy Rev. 2020;133:110338. https://doi.org/10.1016/j.rser.2020.110338
48. Boonchuay P, Wongpoomchai R, Jaturasitha S, Mahatheeranont S, Watanabe M, Chaiyaso T. Prebiotic properties, antioxidant activity, and acute oral toxicity of xylooligosaccharides derived enzymatically from corncob. Food Biosci. 2021;40:100895. https://doi.org/10.1016/j.fbio.2021.100895
49. Farfan JA. Química de proteínas: aplicada à ciência e tecnologia dos alimentos. 2. ed. Campinas (SP): Editora da UNICAMP; 1994.
50. Lehninger TM, Nelson DL, Cox MM. Princípios de bioquímica. 6. ed. Editora Artmed; 2014

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Derechos de autor 2023 Revista del Instituto Adolfo Lutz