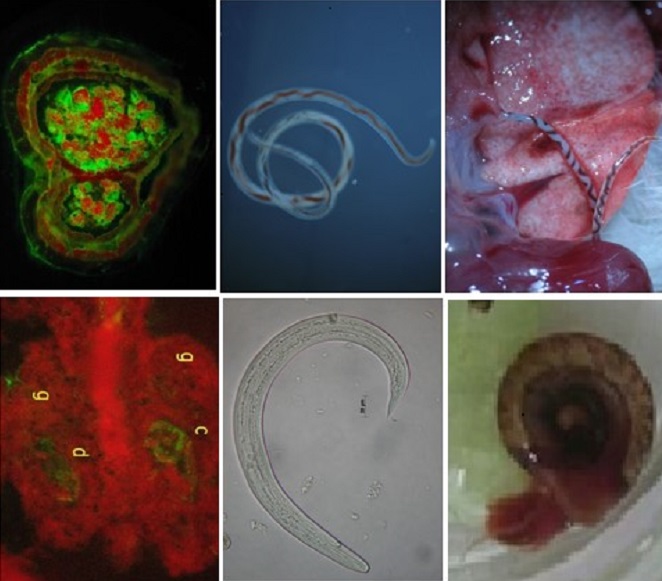
Abstract
The Indirect Immunofluorescence Assay (IFA) was used to identify stage-specific antigenic structures in paraffin sections of female larvae and worms and snails tissues, infected with third stage larvae of Angiostrongylus cantonensis. Sera from eosinophilic meningitis cases were used to assess reactivity. Non-reactive sera from patients with other parasitic diseases and from individuals without other etiologies were used as controls for cross-reactivity. Larvae and worms showed high reactivity to IgG antibodies. IgM antibodies reacted with low intensity only to larvae. Fluorescent reactions were observed in the cuticles and internal structures on worms sections, with a marked reaction in the uterus content. In the snail tissues, the larvae were found exclusively inside the granulomas, with fluorescent markings in the cuticles of the larvae and inside the granulomatous tissues. This fluorescent pattern suggests the presence of excretory/secretory antigens distributed throughout the granulomas. Expressive cross reactivity occurred in sera from patients with other parasitic diseases, especially strongyloidiasis. The use of IFA applied to paraffin sections to identify structures with antigenic potential and the study of new serological markers, can contribute to the improvement of the laboratory diagnosis of eosinophilic meningitis.
References
1. Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR. Human angiostrongyliasis. Lancet Infect Dis. 2008;8(10):621-30. https//:doi.org/10.1016/S1473-3099(08)70229-9
2. Graeff-Teixeira C, Morassutti AL, Jones MK. Diagnosing and understanding angiostrongyliasis, a zoonotic cause of meningitis. ACS Chem Neurosci. 2018;21;9(3):393-4. https//:doi.org/10.1021/acschemneuro.8b00018
3. Kramer K J, Posner J, Gosnell W L. Role of gastropod mucus in the transmission of Angiostrongylus cantonensis, a potentially serious neurological infection. ACS Chem Neurosci. 2018;9(4):629-32. https://doi.org/10.1021/acschemneuro.7b00491
4. Graeff-Teixeira C, da Silva AC, Yoshimura K. Update on eosinophilic meningoencephalitis and its clinical relevance. Clin Microbiol Rev. 2009;22(2):322-48, Table of Contents. https://doi.org/10.1128/CMR.00044-08
5. Feng Y, Zheng C, Zhou Z, Xiong H, Feng F, Xie F et al. IL-17A neutralizing antibody attenuates eosinophilic meningitis caused by Angiostrongylus cantonensis by involving IL-17RA/Traf6/NF-κB signaling. Exp Cell Res. 2019;384(1):111554. https://doi.org/10.1016/j.yexcr.2019.111554
6. Lv S, Zhou XN, Andrews JR. Eosinophilic meningitis caused by Angiostrongylus cantonensis. ACS Chem Neurosci. 2017;8(9):1815-6. https://doi.org/10.1021/acschemneuro.7b00233
7. Morassutti AL, Thiengo SC, Fernandez M, Sawanyawisuth K, Graeff-Teixeira C. Eosinophilic meningitis caused by Angiostrongylus cantonensis: an emergent disease in Brazil. Mem Inst Oswaldo Cruz. 2014;109(4):399-407. https://doi.org/10.1590/0074-0276140023
8. Espírito-Santo MC, Pinto PL, Mota DJ, Gryschek RC. The first case of Angiostrongylus cantonensis eosinophilic meningitis diagnosed in the city of São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2013;55(2):129-32. https://doi.org/10.1590/s0036-46652013000200012
9. Barbosa TA, Thiengo SC, Fernandez MA, Graeff-Teixeira C, Morassutti AL, Mourão FRP et al. Infection by Angiostrongylus cantonensis in both humans and the snail Achatina (Lissachatina) fulica in the city of Macapá, in the Amazon Region of Brazil. Mem Inst Oswaldo Cruz. 2020;115:e200115. https://doi.org/10.1590/0074-02760200115
10. da Mota DJG, de Melo LCV, Pereira-Chioccola VL, Gava R, Pinto PLS. First record of natural infection by Angiostrongylus cantonensis (Nematoda: Metastrongyloidea) in Belocaulus willibaldoi and Rattus norvegicus in an urban area of São Paulo city, SP, Brazil. Heliyon. 2020;6(10):e05150. https://doi.org/10.1016/j.heliyon.2020.e05150
11. Souza FN, Aguiar Santos M, Almeida Alves D, Cecília Vieira de Melo L, Jessé Gonçalves da Mota D, Cristina Pertile A et al. Angiostrongylus cantonensis in urban populations of terrestrial gastropods and rats in an impoverished region of Brazil. Parasitology. 2021;148(8):948-1002. https://doi.org/10.1017/S0031182021000597
12. Wang QP, Wu ZD, Wei J, Owen RL, Lun ZR. Human Angiostrongylus cantonensis: an update. Eur J Clin Microbiol Infect Dis. 2012;31(4):389-95. https://doi.org/10.1007/s10096-011-1328-5
13. Morassutti AL, Levert K, Perelygin A, da Silva AJ, Wilkins P, Graeff-Teixeira C. The 31-kDa antigen of Angiostrongylus cantonensis comprises distinct antigenic glycoproteins. Vector Borne Zoonotic Dis. 2012;12(11):961-8. https://doi.org/10.1089/vbz.2011.0957
14. Song Z, Huang H, Tan F, Zhang E, Hu J, Pan C. Differential proteomics analysis of female and male adults of Angiostrongylus cantonensis. Exp Parasitol. 2012;131(2):169-74. https://doi.org/10.1016/j.exppara.2012.03.019
15. Chen KY, Lu PJ, Cheng CJ, Jhan KY, Yeh SC, Wang LC. Proteomic analysis of excretory-secretory products from young adults of Angiostrongylus cantonensis. Mem Inst Oswaldo Cruz. 2019;114:e180556. https://doi.org/10.1590/0074-02760180556
16. Eamsobhana P, Yong HS. Immunological diagnosis of human angiostrongyliasis due to Angiostrongylus cantonensis (Nematoda: Angiostrongylidae). Int J Infect Dis. 2009;13(4):425-31. https://doi.org/10.1016/j.ijid.2008.09.021
17. Morassutti AL, Rascoe LN, Handali S, DA Silva AJ, Wilkins PP, Graeff-Teixeira C. Cross-reactivity of the 31 kDa antigen of Angiostrongylus cantonensis – Dealing with the immunodiagnosis of meningoencephalitis. Parasitology. 2017;144(4):459-63. https://doi.org/10.1017/S0031182016001918
18. Rugai E, Mattos T, Brisola AP. Nova técnica para isolar larvas de nematóides das fezes - Modificação do Método de Baermann. Rev Inst Adolfo Lutz. 1954;14(1):5-8. [cited 2021 Jun 17]. Avaiable from: https://periodicos.saude.sp.gov.br/index.php/RIAL/article/view/33246
19. Deelder AM, Kornelis D. A comparison of the IFA and the ELISA for the demonstration of antibodies against schistosome gut-associated polysaccharide antigens in schistosomiasis. Z Parasitenkd. 1980;64(1):65-75. https://doi.org/10.1007/BF00927057
20. Michalany J. Técnica Histológica em Anatomia Patológica: com instruções para o cirurgião, enfermeira e citotécnico. 3.ed. São Paulo: Editora Michalany; 1998.296p.
21. Pan, CT. The general histology and topographic microanatomy of Australorbis glabratus. Bull. Mus. Comp. Zool. 1958; 119(3):237-59. [cited 2021 Jun 28]. Avaiable from: https://ia800204.us.archive.org/4/items/biostor-854/biostor-854.pdf
22. Kanamura HY, Silva RM, Chiodelli SG, Glasser CM, Dias LC. IgM-immunofluorescence test as a diagnostic tool for epidemiologic studies of Schistosomiasis in low endemic areas. Mem Inst Oswaldo Cruz. 2002;97(4):485-9. https://doi.org/10.1590/s0074-02762002000400005
23. Voller A, De Savigny D. Diagnostic serology of tropical parasitic diseases. J Immunol Methods. 1981;46(1):1-29. https://doi.org/10.1016/0022-1759(81)90328-8
24. Nash TE. Antibody response to a polysaccharide antigen present in the schistosome gut. I. Sensitivity and specificity. Am J Trop Med Hyg. 1978;27(5):939-43. https://doi.org/10.4269/ajtmh.1978.27.939
25. Deelder AM, van Zeyl RJ, Fillié YE, Rotmans JP, Duchenne W. Recognition of gut-associated antigens by immunoglobulin M in the indirect fluorescent antibody test for schistosomiasis mansoni. Trans R Soc Trop Med Hyg. 1989;83(3):364-7. https://doi.org/10.1016/0035-9203(89)90506-3
26. Andrade ZA, Sadigursky M. Estudos sobre um antígeno polissacáride do Schistosoma mansoni. Mem Inst Oswaldo Cruz. 1980;75(1-2):47-56. https://doi.org/10.1590/s0074-02761980000100005
27. Bender AL, Maurer RL, da Silva MC, Ben R, Terraciano PB, da Silva AC et al. Ovos e órgãos reprodutores de fêmeas de Angiostrongylus costaricensis são reconhecidos mais intensamente por soros humanos de fase aguda na angiostrongilíase abdominal. Rev Soc Bras Med Trop. 2003;36(4):449-54. https://doi.org/10.1590/s0037-86822003000400003
28. Harris KR, Cheng TC. The encapsulation process in Biomphalaria glabrata experimentally infected with the metastrongylid Angiostrongylus cantonensis: light microscopy. Int J Parasitol. 1975;5(5):521-8. https://doi.org/10.1016/0020-7519(75)90044-2
29. Dzik JM. Molecules released by helminth parasites involved in host colonization. Acta Biochim Pol. 2006;53(1):33-64.
30. Oku Y, Kamiya M. Circumoval and circumlarval precipitate reactions of Angiostrongylus cantonensis. Jpn J Vet Res. 1984;32(1):23-39.
31. Chen MX, Zhang RL, Chen JX, Chen SH, Li XH, Gao ST et al. Monoclonal antibodies against excretory/secretory antigens of Angiostrongylus cantonensis. Hybridoma (Larchmt). 2010;29(5):447-52. https://doi.org/10.1089/hyb.2010.0008
32. Shih HH, Chen SN. Immunodiagnosis of angiostrongyliasiswith monoclonal antibodies recognizing a circulating antigen of mol. wt 91,000 from Angiostrongylus cantonensis. Int J Parasitol. 1991;21:171-7. https://doi.org/10.1016/0020-7519(91)90007-T
33. Lai SC, Jiang ST, Chen KM, Lee HH. Matrix metalloproteinases activity demonstrated in the infective stage of the nematodes, Angiostrongylus cantonensis. Parasitol Res. 2005;97(6):466-71. https://doi.org/10.1007/s00436-005-1484-6
34. Lee JD, Yen CM. Protease secreted by the infective larvae of Angiostrongylus cantonensis and its role in the penetration of mouse intestine. Am J Trop Med Hyg. 2005;72(6):831-6.
35. Morassutti AL, Levert K, Pinto PM, da Silva AJ, Wilkins P, Graeff-Teixeira C. Characterization of Angiostrongylus cantonensis excretory-secretory proteins as potential diagnostic targets. Exp Parasitol. 2012;130(1):26-31. https://doi.org/10.1016/j.exppara.2011.10.003
36. Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;23;291(5512):2370-6. https://doi.org/10.1126/science.291.5512.2370
37. Veríssimo CM, Morassutti AL, von Itzstein M, Sutov G, Hartley-Tassell L, McAtamney S et al. Characterization of the N-glycans of female Angiostrongylus cantonensis worms. Exp Parasitol. 2016;166:137-43. https://doi.org/10.1016/j.exppara.2016.04.012
38. Morassutti AL, Levert K, Perelygin A, da Silva AJ, Wilkins P, Graeff-Teixeira C. The 31-kDa antigen of Angiostrongylus cantonensis comprises distinct antigenic glycoproteins. Vector Borne Zoonotic Dis. 2012;12(11):961-8. https://doi.org/10.1089/vbz.2011.0957

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2022 Instituto Adolfo Lutz Journal