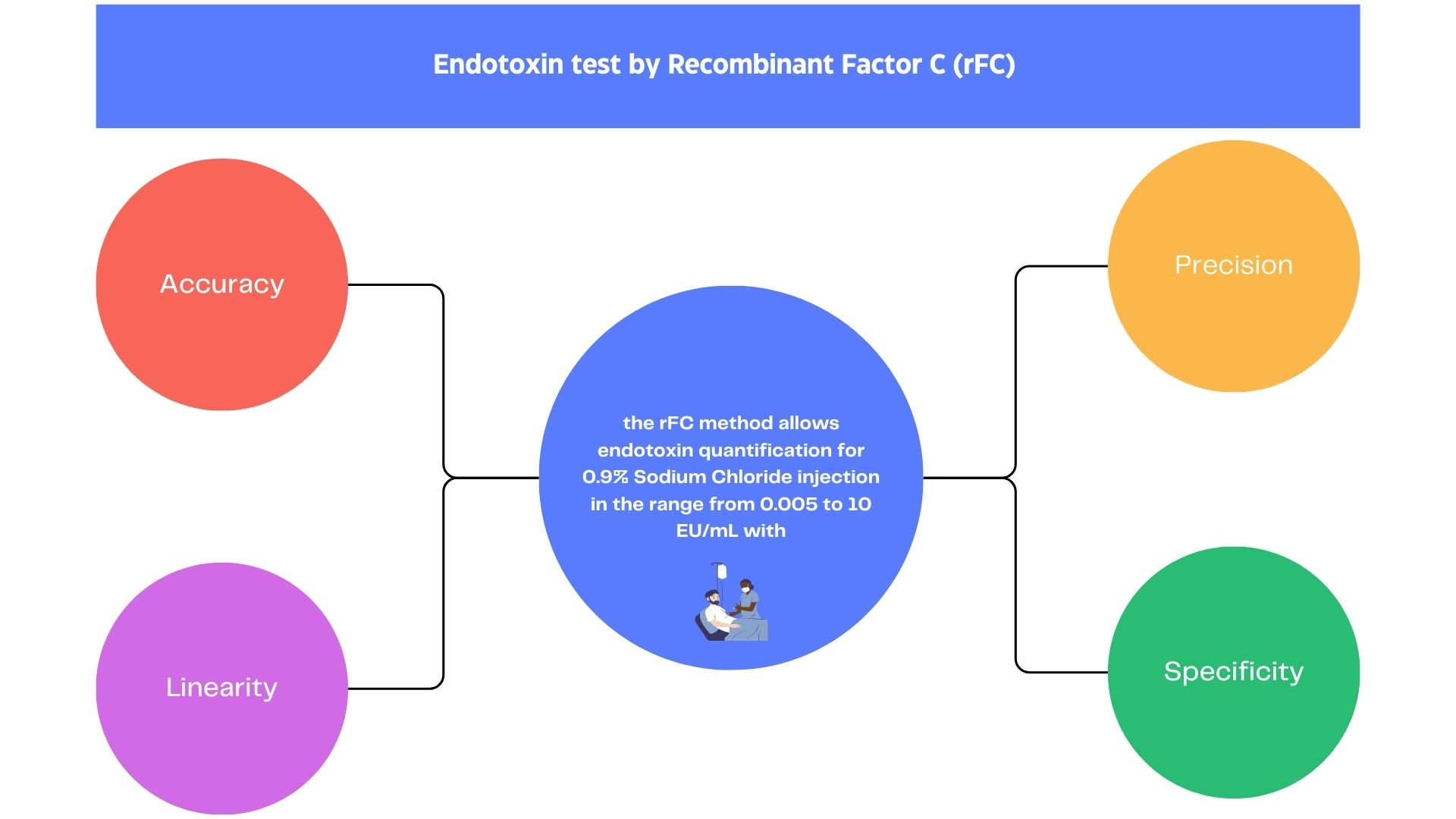
Abstract
Endotoxin contamination is a threat to the safety of pharmaceutical products, especially parenteral drugs. Any sterile and/or pyrogen-free pharmaceutical product requires regulatory specifications to ensure safe patient use. This study covers theperformance evaluation study of an endotoxinquantitat ion commercial kit by recombinant Factor C (rFC),Endozyme II® Go, for 0.9% sodium chloride injection. The samples were spiked with endotoxin solutions between 0.0005 and 10 EU/mL and tested by the rFC kit to evaluate precision, accuracy, detection and quantification limits, linearity, and robustness. Each of the six points was assayed at least five times. The relative standard deviation for precision testing ranged from 1.9 to 8.3%. The recovery accuracy values of endotoxin were between 61% and 125% for the range from 0.005 to 10 EU/mL. The results demonstrated that the rFC method allows endotoxin quantification with accuracy, precision, specificity, and linearity for the range of 0.005 and 10 EU/mL for 0.9% sodium chloride injection.
References
Bolden JS, Warburton RE, Phelan R, Murphy M, Smith KR, De Felippis MR, et al. Endotoxin recovery using limulus amebocyte lysate (LAL) assay. Biologicals. 2016;44:434–40. https://doi.org/10.1016/j.biologicals.2016.04.009
Schletter J, Heine H, Ulmer AJ, Rietschel ET. Molecular mechanisms of endotoxin activity. Arch. Microbiol. 1995;164:383–89. https://doi.org/10.1007/BF02529735
DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci. Biobehav. 2010;34:130–143. https://doi.org/10.1016/j.neubiorev.2009.07.014
Sandle T. Characterizing the Microbiota of a pharmaceutical water system - A metadata study. SOJ Microbiol. Infect. Dis. 2015;3:01–08. https://doi.org/10.15226/sojmid/3/2/00133
United States Pharmacopeia - USP. <1223> Validation of alternative microbiological methods. Rockville: The United States Pharmacopeial Convention, 2023. https://doi.org/10.31003/USPNF_M99943_03_01
Hasiwa N. Evidence for the detection of non-endotoxin pyrogens by the whole blood monocyte activation test. ALTEX. 2013;30:169–208. https://doi.org/10.14573/altex.2013.2.169
Greenhough J. Life cycle of an analytical method - A case study on the monocyte activation test. [publisher unknown]. 1–6. Available from: https://wickhammicro.co.uk/Content/Downloads/Wickham-Laboratories-Case-Study-on-the-Monocyte-Activation-Test.pdf
European Medicines Agency - EMA. Guideline on the replacement of rabbit pyrogen testing by an alternative test for plasma derived medicinal products. 2009. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-replacement-rabbit-pyrogen-testing-alternative-test-plasma-derived-medicinal-products_en.pdf
Apparao P, Shashidher B, Ajay KP. Monocyte activation test : A new pharmacoepial quality control test for pyrogens - A Review. J. Adv. Pharm. Sci. 2011;1:122–131
Bolden JS, Claerbout ME, Miner MK, Murphy MA, Smith KR, Warburton RE. Evidence Against a bacterial endotoxin masking effect in biologic drug products by limulus amebocyte lysate detection. PDA J. Pharm. Sci. Technol. 2014;68:472–477. https://doi.org/10.5731/pdajpst.2014.00999
Maloney T, Phelan R, Simmons N. Saving the horseshoe crab: A synthetic alternative to horseshoe crab blood for endotoxin detection. PLoS Biol. 2018;16:1–10. https://doi.org/10.1371/journal.pbio.2006607
Russel MS, Burch RL. The principles of humane experimental technique. Med. J. Aust. 1960;1:500-500. https://doi.org/10.5694/j.1326-5377.1960.tb73127.x
Peterbauer A, Werner ER, Werner-Fermaver G. Further development of a cell culture model for the detection of bacterial pyrogens. ALTEX. 1999;16:3–8
Peterbauer A, Eperon S, Jungi TW, Werner ER, Werner-Felmayer G. Interferon-γ-primed monocytoid cell lines: optimizing their use for in vitro detection of bacterial pyrogens, J. Immunol. Methods. 2000;233:67–76. https://doi.org/10.1016/S0022-1759(99)00189-1
Schindler S, Spreitzer I, Löschner B, Hoffmann S, Hennes K, Halder M, et al. International validation of pyrogen tests based on cryopreserved human primary blood cells, J. Immunol. Methods. 2006;316:42–51. https://doi.org/10.1016/j.jim.2006.07.023
Utescher CLA, Buosi KL, Botosso VF, Quintilio W. Monocyte activation test (MAT) as a possibility of replacement for the rabbit pyrogen test in hyperimmune sera. Brazilian J. Pharm. Sci. 2018;54:3–8. https://doi.org/10.1590/s2175-97902018000217530
Ding JL, Chai C, Pui AWM, Ho B. Expression of full length and deletion homologues of Carcinoscorpius rotundicauda Factor C in Saccharomyces cerevisiae: Immunoreactivity and endotoxin binding, Innate Immun. 1997;4:33–43. https://doi.org/10.1177/096805199700400105
Ding, JL, Ho B. Endotoxin detection - From limulus amebocyte lysate to recombinant factor C. In: Quinn PJ, Xiaoyuan W (Eds.). Endotoxins Struct. Funct. Recognit. Netherlands: Springers; 2010. p. 187–208. https://doi.org/10.1007/978-90-481-9078-2_9
Bolden J, Smith K. Application of recombinant Factor C reagent for the detection of bacterial endotoxins in pharmaceutical products. PDA J. Pharm. Sci. Technol. 2017;71:405–12. https://doi.org/10.5731/pdajpst.2017.007849
Bolden J, Knight M, Stockman S, Omokoko B. Results of a harmonized endotoxin recovery study protocol evaluation by 14 BioPhorum Operations Group (BPOG) member companies. Biologicals. 2017;48:74–81. https://doi.org/10.1016/j.biologicals.2017.05.003
Marius M, Vacher F, Bonnevay T. Comparison of limulus amoebocyte lysate and recombinant factor C assays for endotoxin detection in four human vaccines with complex matrices. PDA J. Pharm. Sci. Technol. 2020;74:394–407. https://doi.org/10.5731/pdajpst.2019.010389
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Validation of analytical procedures: text and methodology Q2(R1). [place unknown]; [publisher unknown]; 2005. Available from: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf
European Pharmacopoeia. 2.6.32 Test for bacterial endotoxins using recombinant factor C. 10.3 ed. Strasbourg: Council of Europe, 2021.
European Pharmacopoeia. 2.6.14 Bacterial endotoxins. 11.0 ed. Strasbourg: Council of Europe, 2022.
European Pharmacopoeia. Monographs: Water for injections (0169). 11.4 ed. Strasbourg: Council of Europe, 2023.
European Pharmacopoeia. Monographs: Purified Water (0008). 11.4 ed. Strasbourg: Council of Europe, 2023.
BioMérieux. Endozyme II GoTM: rapid detection of endotoxins by recombinant factor. C049458-01. Munchen: BioMérieux; 2018.
Marius M, Vacher F, Bonnevay T. Comparison of bacterial endotoxin testing methods in purified pharmaceutical water matrices. Biologicals. 2020;67:49–55. https://doi.org/10.1016/j.biologicals.2020.07.001
United States Pharmacopeia - USP. <86> Bacterial endotoxins test using recombinant reagents. In: Pharmacopeial Forum 49(6). Rockville: The United States Pharmacopeial Convention, 2023. Available from: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/usp-nf-notices/86-bacterial-endotoxins-tests-using-recombinant-reagents.pdf

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 Ellen Hilinski, Daniela Dal Molim Ghisleni, Carla Lilian de Agostini Utescher, Wagner Quintilio , Adriana Aparecida Buzzo Almodovar , Adriana Bugno, Terezinha de Jesus Andreoli Pinto