Resumo
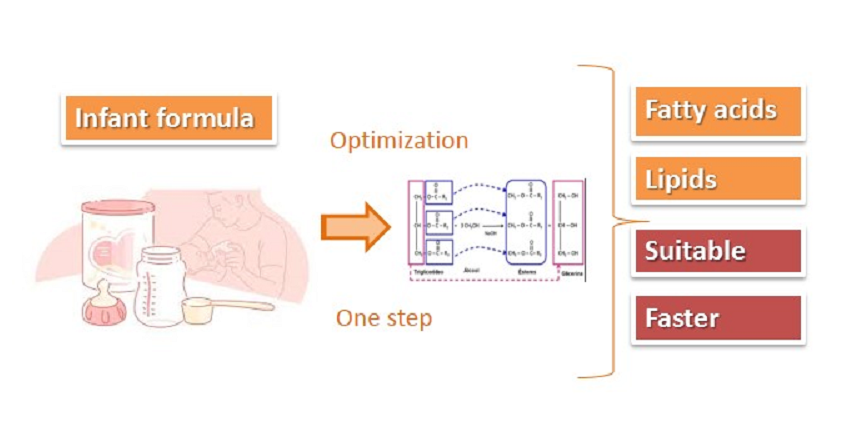
Lipids in food are conventionally analyzed in two stages: extraction with organic solvent and fat esterification reaction, in this case, the type of fat of each food influences the choice of extraction and esterification reagents. In the direct method, such procedures are performed in one step. This work compared the conventional extraction method and quantification of lipids and fatty acids, with a direct method in infant formula. A reference sample of infant formula conteining certified lipids and fatty acids values from the National Institute of Standards and Technology was used. The conventional method for lipid analysis used the acid hydrolysis method; for the determination of fatty acids, the fats were extracted with a mixture of ethyl ether and petroleum ether. The direct method consisted of direct trans esterification with sodium methoxide. In the analysis of fatty acids, the majority of the results showed statistically equal values (α < 0.05) for both methods. The direct method proved suitable, mainly because of reduction in analytical time and quantity of solvents.
Referências
1. Kus MMM, Aued-Pimentel S, Mancini-Filho J. Comparação de métodos analíticos para determinação de lipídios e ácidos graxos polinsaturados por cromatografia gasosa em fórmula infantil. Rev Inst Adolfo Lutz. 2009;68(1):12-20. Available from: https://periodicos.saude.sp.gov.br/index.php/RIAL/article/view/32737
2. Kus MMM, Silva SA, Aued-Pimentel S, Mancini-Filho J. Informação nutricional de fórmulas infantis comercializadas no Estado de São Paulo: avaliação dos teores de lipídeos e ácidos graxos. Rev Nutr. 2011;24(2):209-18. https://doi.org/10.1590/S1415-52732011000200002
3. Kus MMM, Aued-Pimentel S, Mancini-Filho J. Estabilidade dos ácidos graxos poli-insaturados presentes em fórmulas infantis comerciais. Braz J Food Tech. 2011;14(2):145-53. Available from: http://bjft.ital.sp.gov.br/arquivos/artigos/v14n2457a.pdf
4. Aued-Pimentel S. Avaliação de procedimentos analíticos para a determinação de lipídios e ácidos graxos em produtos alimentícios [tese de doutorado]. São Paulo (SP): Coordenadoria de Controle de Doenças da Secretaria de Estado da Saúde de São Paulo; 2007.
5. Official Methods of Analysis of AOAC. 21. ed., Gaithersburg: AOAC International, 2019. [Accessed on 2019 Jun 18]. Available from: http://www.eoma.aoac.org/
6. Adrian J, Potus J, Poiffait A, Dauvillier P. Análisis nutricional de los alimentos. Métodos fisico químicos generales. Zaragosa: Editorial Acribia; 2000.
7. Christie WW. Preparation of esters derivatives of fatty acids for chromatographic analysis. In: Advances in lipid methodology – two, 1993; 2: 69-111. [Accessed on 2006 Out 05]. Available from: https://lipidlibrary.aocs.org/lipid-analysis/selected-topics-in-the-analysis-of-lipids/preparation-ofester-derivatives-of-fatty-acids-for-chromatographic-analysis
8. Carpenter DM, Ngeh-Ngwainbi J, Lee S. Lipid Analysis. In: Carpenter DE, Sullivan DM, organizer. Methods of analysis for nutritional labeling. Arlington: AOAC International; 1993. p.85-104.
9. Carrapiso AI, Garcia C. Development in lipid analysis: some new extraction techniques and in situ transesterification. Lipids. 2000;35(11):1167-77 https://doi.org/10.1007/s11745-000-0633-8
10. Golay PA, Dionisi F, Hug B, Giuffrida F, Destaillats F. Direct quantification of fatty acids in dairy powders with special emphasis on trans fatty acids content. Food Chem. 2006;101(3): 1115-20. https://doi.org/10.1016/j.foodchem.2006.03.011
11. Castro-Gómez P, Fontecha J, Rodríguez-Alcalá LM. A high-performance direct transmethylation method for total fatty acids assessment in biological and foodstuff samples. Talanta. 2014;128:518-23. https://doi.org/10.1016/j.talanta.2014.05.051
12. Liu Z, Wang J, Li C, Rochfort S. Development of one-step sample preparation methods for fatty acid profiling of milk fat. Food Chem. 2020;315:126281. http://dx.doi.org/10.1016/j.foodchem.2020.126281
13. O’Fallon JV, Busboom JR, Nelson ML, Gaskins, CT. A direct method for fatty acid methyl ester synthesis: application to wet meat tissues, oils, and feedstuffs. J Anim Sci. 2007; 85(6):1511-21. https://doi.org/10.2527/jas.2006-491
14. Cruz-Hernandez C, Goeuriot S, Giuffrida F, Thakkar SK, Destaillats F. Direct quantification of fatty acids in human milk by gas chromatography. J Chromatogr A. 2013;1284:174-9. https://doi.org/10.1016/j.chroma.2013.01.094
15. Abdulkadir S, Tsuchiya M. One-step method for quantification and qualitative analysis of fatty acids in marine animal samples. J Exp Mar Biol Ecol. 2008;354:1-8. https://doi.org/10.1016/j.jembe.2007.08.024
16. Wang Y, Sunwoo H, Cherian G, Sim JS. Fatty acid determination in chicken egg yolk: a comparison of different methods. Poult Sci. 2000; 79(8):1168-71. https://doi.org/10.1093/ps/79.8.1168
17. Mazalli MR, Bragagnolo N. Validation of two methods for fatty acids analysis in eggs. Lipids. 2007;42(5):483-90. https://doi.org/10.1007/s11745-007-3046-4
18. Ehimen EA, Sun ZF, Carrington CG. Variables affecting the in situ transesterification of microalgae lipids. Fuel. 2010;89(3):677-84. https://doi.org/10.1016/j.fuel.2009.10.011
19. Velasquez-Orta SB, Lee JGM, Harvey A. Alkaline in situ transesterification of Chlorella vulgaris. Fuel. 2012;94:544-50. https://doi.org/10.1016/j.fuel.2011.11.045
20. Official Methods and Recommended Practices of the AOCS. Revisions 2017. 7. ed., Champaign: American Oil Chemists’ Society, 2017. [Accessed on 2018 Nov 14]. Available from: https://www.aocs.org/attain-lab-services/methods/methods
21. Ludbrook J. Confidence in Altman-Bland plots: a critical review of the method of differences. Clin Exp Pharmacol Physiol. 2010;37(2):143-9. https://doi.org/10.1111/j.1440-1681.2009.05288.x
22. ABNT NBR ISO/IEC 17025. Requisitos gerais para a competência de laboratórios de ensaio e calibração; 2017.
23. Melo EDA. O uso dos MRC (Materiais de Referência Certificados) na avaliação dos métodos analíticos. Rev Analytica. 2020. [Accessed on 2021 Jun 10]. Available from: https://revistaanalytica.com.br/o-uso-dos-mrc-materiais-de-referencia-certificados-na-validacao-dosmetodos-analiticos/#_edn1
24. Hartman L, Lago RAC. Rapid preparation of fatty acid methyl esters from lipids. Lab Prac. 1973;22(6):475-6. Available from: https://www.scienceopen.com/document?vid=5334de68-6e03-446b-85e7-244d0ac1ce1b
25. Maia EL, Rodrigues-Amaya DBR. Avaliação de um método simples e econômico para a metilação de ácidos graxos com lipídios de diversas espécies de peixes. Rev Inst Adolfo Lutz. 1993;53(1-2):27-35. Available from: https://periodicos.saude.sp.gov.br/index.php/RIAL/article/view/35982
26. Kramer JKG, Blackadar CB, Zhou J. Evaluation of two GC columns (60-m SUPELCOWAX 10 and 100-m CP sil 88) for analysis of milkfat with emphasis on CLA, 18:1, 18:2 and 18:3 isomers, and short- and long-chain FA. Lipids. 2002;37(8):823-35. https://doi.org/10.1007/s11745-002-0967-2
27. Ackman RG, Sipos JC. Application of specific response factors in the gas chromatographic analysis of methyl esters of fatty acids with flame ionization detector. J Am Oil Chem Soc. 1964;41(5):377-8. https://doi.org/10.1007/BF02654818
28. Bannon CD, Graske JD, Hillker AE. Analysis of fatty acid methyl esters with high accuracy and reliability: Validation of theoretical relative response factors of unsaturated esters in the flame ionization detector. J Am Oil Chem Soc. 1986;63(1):105-10. https://doi.org/10.1007/BF02676134
29. Instituto Adolfo Lutz (São Paulo – Brasil). Métodos físico-químicos para análise de alimentos: normas analíticas do Instituto Adolfo Lutz. 4. ed. [1. ed. digital]. São Paulo (SP): Instituto Adolfo Lutz; 2008. Available from: http://www.ial.sp.gov.br/resources/editorinplace/ial/2016_3_19/analisedealimentosial_2008.pdf
30. Instituto Nacional de Metrologia, Qualidade e Tecnologia. Guia de Orientação sobre validação de métodos analíticos. DOQ-CGCRE-0008 – revisão 08. 2020. [Accessed on 2021 Jun10]. Available from: http://www.inmetro.gov.br/credenciamento/organismos/doc_organismos.asp?torganismo=calibensaios
31. National Institute of Standards e Technology (NIST). Department of Commerce. United States of America. Certificate of Analysis – Standard Reference Material 1849 Infant/Adult Nutritional Formula. 2010. [Accessed on 2021 Jun 10]. Available from: https://www-s.nist.gov/srmors/certificates/archives/1849.pdf
32. Hirayama KB, Speridião PGL, Fagundes-Neto U. Ácidos graxos poli-insaturados de cadeia longa. Elect J Ped Gastroent Nutr Liver Dis. 2006;10(3):1-10.
33. Currtis JM, Berrigan N, Dauphinee P. The determination of n-3 fatty acid levels in food products containing microencapsulated fish oil using the one-step extraction method. Part 1: measurement in the raw ingredient and dry powdered foods. J Am Oil Chem Soc. 2008;85(4):297-305. https://doi.org/10.1007/s11746-008-1194-1
34. Schreiner M. Quantification of long chain polyunsaturated fatty acids by gas chromatography: evaluation of factors affecting accuracy. J Chromatogr A. 2005;1095(1-2): 126-30. https://doi.org/10.1016/j.chroma.2005.07.104
35. National Institute of Standards e Technology (NIST). Department of Commerce. United States of America. NIST Special Publication 260-136 – Standard Reference Materials Definitions of Terms and Modes Used at NIST for Value-Assignment of Reference Materials for Chemical Measurements. 2000. [Available from: 2021 Jun 10]. Available from: https://www.nist.gov/system/files/documents/srm/SP260-136.PDF
36. Maduko CO, Park YW, Akoh CC. Characterization and oxidative stability of structured lipids: infant milk fat analog. J Am Oil Chem Soc. 2008;85(3):197-204. https://doi.org/10.1007/s11746-007-1192-8
37. Twomey PJ, Kroll MH. How to use linear regression and correlation in quantitative method comparison studies. Int J Clin Pract. 2008;62(4):529-38. https://doi.org/10.1111/j.1742-1241.2008.01709.x

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Copyright (c) 2022 Mahyara Markievicz Mancio Kus-Yamashita, Sabria Aued-Pimentel, Jorge Mancini-Filho