Abstract
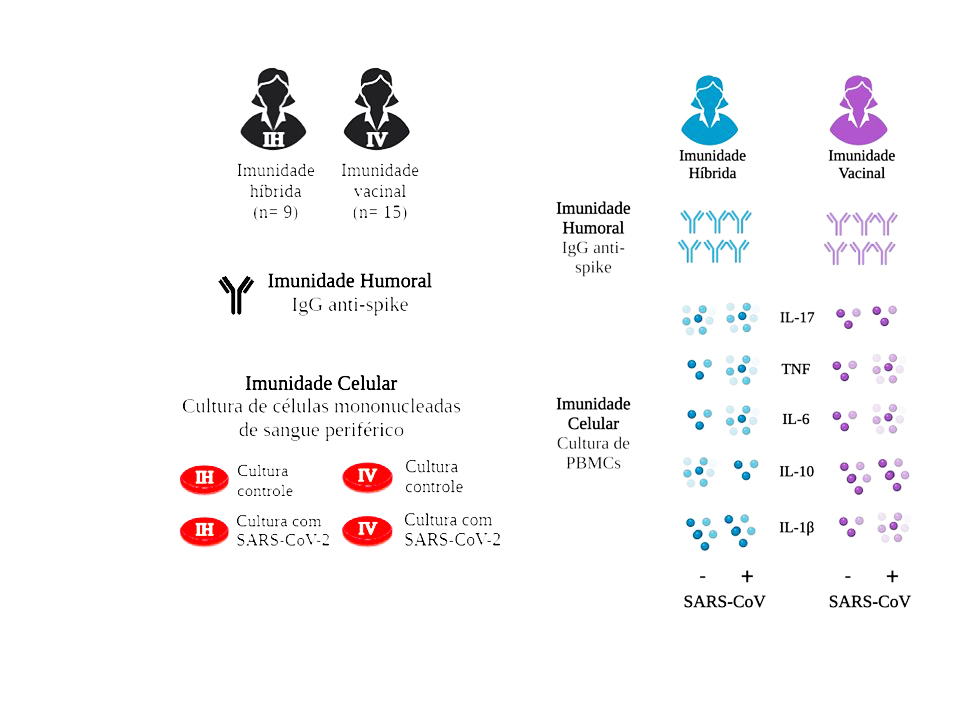
The immune response generated by SARS-CoV-2 vaccination protocols and natural infection remains incompletely understood. We compared individuals who received a heterologous vaccination scheme with a booster shot (vaccine immunity) to those who experienced a mild COVID-19 episode (hybrid immunity) during the same timeframe. Our findings revealed similar levels of SARS-CoV-2 antibodies in both groups. Stimulation by viral antigen in mononuclear cell cultures induced pro-inflammatory cytokines in both groups, while individuals with vaccine immunity exhibited lower IL-17. These results suggest that a vaccine booster can induce an immune response in previously vaccinated individuals comparable to that elicited by natural SARS-CoV-2 infection.
References
Fiolet H, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clinical Microbiology and Infection. 2022;28(2):202-221. https://doi.org/10.1016/j.cmi.2021.10.005
Vespa S, Simeone P, Catitti G, Buca D, Bellis D, Pierdomenico L et al. SARS-CoV-2 and immunity: natural infection compared with vaccination. Int J Mol Sci. 2022;23:8982. https://doi.org/10.3390/ijms23168982
Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARSCoV-2. Lancet Infect Dis. 2021;21(2):e26-e35. https://doi.org/10.1016/S1473-3099(20)30773-8
Rossi C, Lanuti P, Cicalini I, Bellis D, Pierdomenico L, Boccio P et al. BNT162b2 mRNA vaccination leads to long-term protection from COVID-19 disease. Vaccines. 2021;9:1164. https://doi.org/10.3390/vaccines9101164
Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. https://doi.org/10.1056/NEJMoa2114583
Olajide OA, Iwuanyanwu VU, Adegbola OD, Al-Hindawi AA. SARS-CoV-2 spike glycoprotein S1 induces neuroinflammation in BV-2 microglia. Mol Neurobiol. 2022;59(1):445-58. https://doi.org/10.1007/s12035-021-02593-6
Wu X, Xia T, Shin WJ, Yu KM, Jung W, Herrmann A et al. Viral mimicry of Interleukin-17A by SARSCoV-2 ORF8. mBio. 2022;13(2):e00402-22. https://doi.org/10.1128/mbio.00402-22
Udomsinprasert W, Jittikoon J, Sangroongruangsri S, Chaikledkaew U. Circulating levels of Interleukin-6 and Interleukin-10, but not tumor necrosis factor-alpha, as potential biomarkers of severity and mortality for COVID-19: systematic review with meta-Analysis. J Clin Immunol. 2020;41:11-22. https://doi.org/10.1007/s10875-020-00899-z

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 Júlia Bombini Faustini, Ana Paula Campanelli, Thais Fernanda de Campos Fraga da Silva, Vânia Nieto Brito de Souza