Resumen
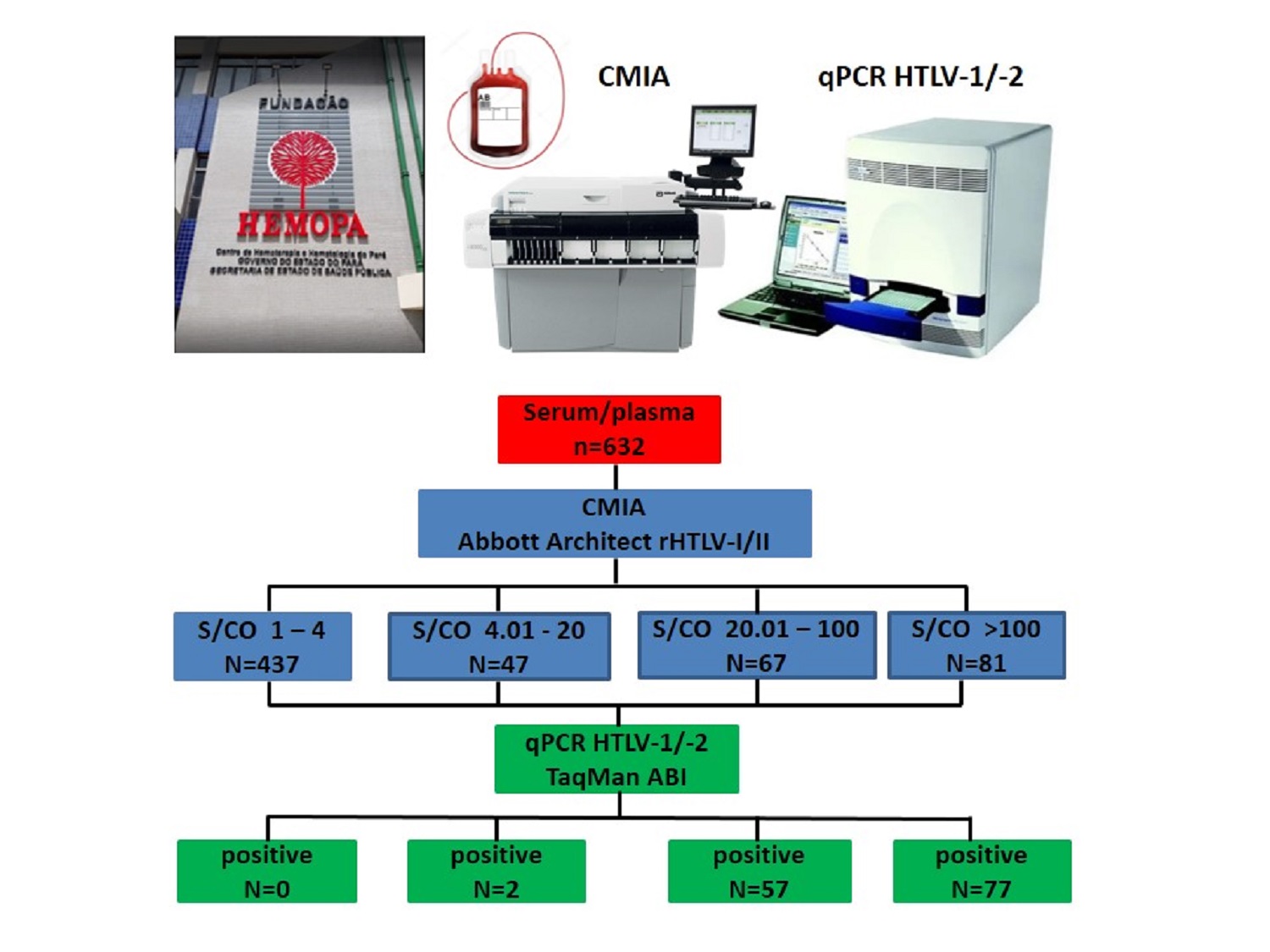
The present study aims to correlate the sample-to-cutoff ratios (S/CO) distributions of reactive results for HTLV-1/2 antibodies with the detection of proviral DNA in a population of blood donor candidates. It was carried out a retrospective data search of 632 HTLV-1/2 reactive samples, submitted to confirmatory testing from January 2015 to December 2019. Serological screening was performed by chemiluminescent microparticle immunoassay Architect rHTLV-I/II, whereas confirmatory testing was performed by in-house real-time polymerase chain reaction method. 496 out of 632 samples (78%) had undetectable HTLV-1/2 proviral DNA and 136 (22%) had detectable proviral DNA. HTLV infection was not confirmed in any individual for whom the S/CO ratio value was <4, and proviral DNA detection rates gradually escalated as S/CO ratio values increased. The sensitivity and predictive positive value found for the Architect rHTLV-I/II was 100% and 22%, respectively. The receiver operating characteristic (ROC) curve analysis showed that the optimal S/CO ratio value for predicting the presence of HTLV-1/2 was 18.11. High S/CO ratios were more associated with the detection of proviral DNA. The S/CO ratio value <4 suggests excluding true HTLV infection and the risk of blood transmission.
Citas
1. International Committee on Taxonomy of Viruses – ICTV. Taxonomy history: primate T-lymphotropic virus 1; 2019. [Accessed on 2022 Mar 23]. Available from: https://talk.ictvonline.org/taxonomy/p/taxonomy-history?taxnode_id=201904999
2. Lima VFS, Torres RM, Guerra FM, Carvalho TL, Magalhães PMR. Human T-cell lymphotropic viruses (HTLV-1 and HTLV-2): literature review. Braz J Health Rev. 2021;4(5):20900-23. https://doi.org/10.34119/bjhrv4n5-193
3. Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. U. S. A. 1980;77(12):7415-9. https://doi.org/10.1073/pnas.77.12.7415
4. Forlani G, Shallak M, Accolla RS, Romanelli MG. HTLV-1 infection and pathogenesis: new insights from cellular and animal models. Int J Mol Sci. 2021;22(15):8001. https://doi.org/10.3390/ijms22158001
5. Martinez MP, Al-Saleem J, Green PL. Comparative virology of HTLV-1 and HTLV-2. Retrovirology. 2019;16(1):21. https://doi.org/10.1186/s12977-019-0483-0
6. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388. https://doi.org/10.3389/fmicb.2012.00388
7. Catalan-Soares B, Carneiro-Proietti ABF, Proietti FA; Interdisciplinary HTLV Research Group. Heterogeneous geographic distribution of human T-cell lymphotropic viruses I and II (HTLV-I/II): serological screening prevalence rates in blood donors from large urban areas in Brazil. Cad Saúde Pública. 2005;21(3):926-31. https://doi.org/10.1590/S0102-311X2005000300027
8. Maneschy CA, Barile KAS, Castro JAA, Palmeira MK, Castro RBH, Amaral CEM. Seroprevalence of the Human T Lymphotropic Virus (HTLV 1 and HTLV 2) in blood donor candidates in the state of Pará, Northern Brazil. Res Soc Dev. 2022;11(4):e1111427082. https://doi.org/10.33448/rsd-v11i4.27082
9. Mendes MFC, Lima JRO, Melo BO, Pinto CMFS, Maia HS, Ferro TAF et al. Molecular detection of human T cell lymphotropic virus type 1 in pregnant women from Maranhão state, Brazil. Braz J Microbiol. 2020;51(2):637-45. https://doi.org/10.1007/s42770-020-00233-0
10. Coordenação Geral de Vigilância das Infecções Sexualmente Transmissíveis: Grupo técnico de especialistas em HTLV. Prevalência da infecção por HTLV-1/2 no Brasil. Boletim Epidemiológico SVS/MS. 2020;51(48):25-33.
11. Maneschy CA, Barile KAS, Castro JAA, Palmeira MK, Castro RBH, Amaral CEM. Epidemiological and molecular profile of blood donors infected with HTLV-1/2 in the state of Pará, northern Brazil. Braz J Microbiol. 2021;52(4):2001-6. https://doi.org/10.1007/s42770-021-00609-w
12. Gross C, Thoma-Kress AK. Molecular mechanisms of HTLV-1 cell-to-cell transmission. Viruses. 2016;8(3):74. https://doi.org/10.3390/v8030074
13. Eusebio-Ponce E, Anguita E, Paulino-Ramirez R, Candel FJ. HTLV-1 infection: an emerging risk. Pathogenesis, epidemiology, diagnosis and associated diseases. Rev Esp Quimioter. 2019;32(6):485-96.
14. Rosadas C, Brites C, Arakaki-Sanchez D, Casseb J, Ishak R. Brazilian Protocol for Sexually Transmitted Infections 2020: human T-cell lymphotropic virus (HTLV) infection. Rev Soc Bras Med Trop. 2021;54(Suppl 1):e2020605. https://doi.org/10.1590/0037-8682-605-2020
15. Busch MP, Bloch EM, Kleinman S. Prevention of transfusion-transmitted infections. Blood. 2019;133(17):1854-64. https://doi.org/10.1182/blood-2018-11-833996
16. Caterino-de-Araujo A, Gonçalves MG. Diagnóstico molecular de vírus T-linfotrópico humano (HTLV): histórico e estado da arte. BEPA, Bol Epidemiol Pauli. 2021;18(212):14-62.
17. Ministério da Saúde (BR). Portaria de consolidação n° 5, de 28 de setembro de 2017. Consolidação das normas sobre as ações e os serviços de saúde do Sistema Único de Saúde. Anexo IV. Diário Oficial da União. Brasília, DF, 2017. Available from: https://www.gov.br/saude/pt-br/composicao/sctie/farmacia-popular%20old/legislacao/prc-5-portaria-de-consolida-o-n-5-de-28-de-setembro-de-2017.pdf/@@download/file/PRC-5-Portaria-deConsolida----o-n---5--de-28-de-setembro-de-2017.pdf
18. Ji H, Chang L, Yan Y, Jiang X, Sun H, Guo F et al. A Strategy for screening and confirmation of HTLV-1/2 infections in Low-Endemic Areas. Front Microbiol. 2020;11:1151. https://doi.org/10.3389/fmicb.2020.01151
19. Andrade RG, Ribeiro MA, Namen-Lopes MSS, Silva SMN, Basques FV, Ribas JG et al. Evaluation of the use of real-time PCR for human T cell lymphotropic virus 1 and 2 as a confirmatory test in screening for blood donors. Rev Soc Bras Med Trop. 2010;43(2):111-15. https://doi.org/10.1590/s0037-86822010000200001
20. Campos KR, Santos FLN, Brito VS, Gonçalves NLS, Araujo THA, Galvão-Castro B et al. Line immunoassay for confirmation and discrimination of human T-cell lymphotropic virus infections in inconclusive western blot serum samples from Brazil. J Clin Microbiol. 2019;58(1):e01384-19. https://doi.org/10.1128/JCM.01384-19
21. Marqué L, Liehl P, De Boer J, Pottel H, Murphy EL, Bruhn R et al. A novel high performing multiplex immunoassay multi-HTLV for serological confirmation and typing of HTLV infections. PLoS Negl Trop Dis. 2021;15(11):e0009925. https://doi.org/10.1371/journal.pntd.0009925
22. Kiely P, Walker K, Parker S, Cheng A. Analysis of sample-to-cutoff ratios on chemiluminescent immunoassays used for blood donor screening highlights the need for serologic confirmatory testing. Transfusion. 2010;50(6):1344-51. https://doi.org/10.1111/j.1537-2995.2009.02572.x
23. Stramer SL, Notari EP, Zou S, Krysztof DE, Brodsky JP, Tegtmeier GE et al. Human T-lymphotropic virus antibody screening of blood donors: rates of false-positive results and evaluation of a potential donor reentry algorithm. Transfusion. 2011;51(4);692-701. https://doi.org/10.1111/j.1537-2995.2010.02903.x
24. Tosswill JHC, Taylor GP. Interpretation of low reactivity in the Abbott Architect rHTLV I/II assay. Transfus Med. 2018;28(4):326-30. https://doi.org/10.1111/tme.12482
25. Tamegão-Lopes BP, Rezende PR, Maradei-Pereira LMC, Lemos JAR. HTLV-1 and HTLV-2 proviral load: a simple method using quantitative real-time PCR. Rev Soc Bras Med Trop. 2006; 39(6):548-52. https://doi.org/10.1590/S0037-86822006000600007
26. Pham D, Nguyen D, Nguyen TA, Tran C, Tran L, Devare S et al. Seroprevalence of HTLV-1/2 among voluntary blood donors in Vietnam. AIDS Res Hum Retroviruses. 2019;35(4):376-81. https://doi.org/10.1089/aid.2018.0240
27. Zhao J, Zhao F, Han W, Xu X, Wang L, Li R et al. HTLV screening of blood donors using chemiluminescence immunoassay in three major provincial blood centers of China. BMC Infect Dis. 2020;20(1):581. https://doi.org/10.1186/s12879-020-05282-2
28. Murphy EL, Cassar O, Gessain A. Estimating the number of HTLV-2 infected persons in the world. Retrovirology. 2015;12(Suppl 1):O5. https://doi.org/10.1186/1742-4690-12-S1-O5
29. Murphy EL, Lee TH, Chafets D, Nass CC., Wang B, Loughlin K et al. Higher human T lymphotropic virus (HTLV) provirus load is associated with HTLV-I versus HTLV-II, with HTLV-II subtype A versus B, and with male sex and a history of blood transfusion. J Infect Dis. 2004;190(3):504-10. https://doi.org/10.1086/422398
30. Waters A, Oliveira AL, Coughlan S, de Venecia C, Schor D, Leite AC et al. Multiplex real-time PCR for the detection and quantitation of HTLV-1 and HTLV-2 proviral load: addressing the issue of indeterminate HTLV results. J Clin Virol. 2011;52(1):38-44. https://doi.org/10.1016/j.jcv.2011.05.022
31. Matsumoto C, Sagara Y, Sobata R, Inoue Y, Morita M, Uchida S et al. Analysis of HTLV-1 proviral load (PVL) and antibody detected with various kinds of tests in Japanese blood donors to understand the relationship between PVL and antibody level and to gain insights toward better antibody testing. J Med Virol. 2017;89(8):1469-76. https://doi.org/10.1002/jmv.24802
32. Yun SG, Kim, SW, Sohn JY, Cho Y. Evaluation of Elecsys HTLV-I/II assay in comparison with ARCHITECT rHTLV-I/II assay with Korean samples. J Clin Lab Anal. 2019;33(6):e22909. https://doi.org/10.1002/jcla.22909
33. Al-Hababi FH, Al-Deailej IM, Al-Sulatan HA, Al-Ghamdi YA, Al-Dossari KM. Human T lymphotropic virus antibodies seroprevalence among healthy blood donors and high risk groups at Riyadh regional laboratory in Riyadh, Saudi Arabia. Saudi Crit Care J. 2020;4(2):73-8. https://doi.org/10.4103/sccj.sccj_13_20
34. Laperche S, Sauleda S, Piron M, Mühlbacher A, Schennach H, Schottstedt V et al. Evaluation of sensitivity and specificity performance of elecsys HTLV-I/II assay in a multicenter study in Europe and Japan. J Clin Microbiol. 2017;55(7):2180-7. https://doi.org/10.1128/JCM.00169-17
35. Brito VS, Santos FLN, Gonçalves NLS, Araujo THA, Nascimento DSV, Pereira FM et al. Performance of commercially available serological screening tests for human T-cell lymphotropic virus infection in Brazil. J Clin Microbiol. 2018;56(12):e00961-18. https://doi.org/10.1128/JCM.00961-18
36. World Health Organization – WHO. Screening donated blood for transfusion-transmissible infections: recommendations. Genebra: World Health Organization; 2010.
Chang L, Ou S, Shan Z, Zhu F, Ji H, Rong X et al. Seroprevalence of human T-lymphotropic virus infection among blood donors in China: a first nationwide survey. Retrovirology. 2021;18(1):2. https://doi.org/10.1186/s12977-020-00546-w
38. Kiely P, Hoad VC, Wood EM. False positive viral marker results in blood donors and their unintended consequences. Vox Sang. 2018;113(6):530-9. https://doi.org/10.1111/vox.12675
39. Vo MT, Bruhn R, Kaidarova Z, Custer BS, Murphy EL, Bloch EM. A retrospective analysis of falsepositive infectious screening results in blood donors. Transfusion. 2016;56(2):457-65. https://doi.org/10.1111/trf.13381
40. Trevethan R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front Public Health. 2017;5:307. https://doi.org/10.3389/fpubh.2017.00307

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Derechos de autor 2022 Revista del Instituto Adolfo Lutz